In this activity we will be using chemically-made electricity in a battery to demonstrate how the flow of electricity through water results in splitting water into its hydrogen and oxygen. This process of passing an electrical current through a solution or substance is called electrolysis.
As a review, Electricity is defined as the flow of electrons from one molecule to another within a substance and can be produced using six different methods. These include:
- Chemical reaction
- Friction
- Heat
- Light
- Magnetism
- Pressure
We power many items from a flashlight to a watch using the chemical reaction in batteries. Before starting this activity, let’s examine what’s inside a battery.
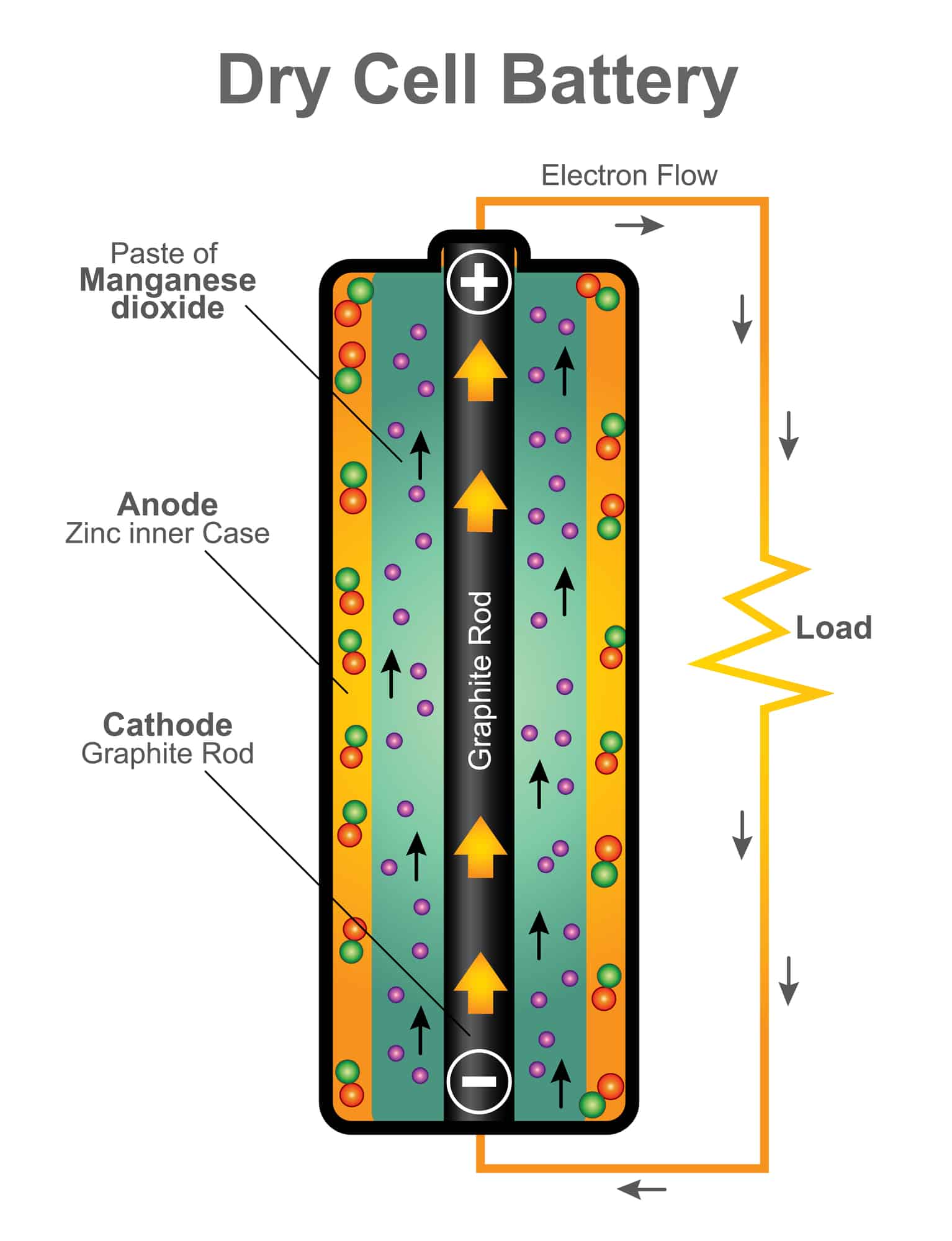
What’s Inside a Battery
According to the Energizer battery company, the following chemicals are inside a 9-volt, AA, AAA, C, and D battery: “…steel and a mix of zinc/manganese/potassium/graphite, with the remaining balance made up of paper and plastic.” See the resources at the end of this post to learn more about how batteries work.
The chemicals in the battery begin to react when you place the battery into a circuit and complete the circuit. For example, in this Build a Circuit post, when the circuit is complete, the LED in the greeting card illuminates.
Another example is when you put batteries into a flashlight, then press the button to turn on the light. The button is pressed in and the circuit is then complete, so the battery powers the flashlight bulb.
Each battery has a positive and negative terminal. When the circuit is complete the electrons created by the chemical reaction flow from the negative end of the battery to the positive end. If a wire is attached the flow of electrons produced by the chemical reaction then flows through the wire or metal that is attached to the positive end of the battery.
In this activity we will be using chemically-made electricity in a battery to split water into hydrogen and oxygen. This process is called electrolysis. We’ll talk more about electrolysis in a bit.
Water is a simple chemical made from two gases — hydrogen and oxygen. Every molecule of water has two atoms of hydrogen for every atom of oxygen. H2O is the chemical formula for a molecule of water.
Let’s see what happens!
Splitting Water into Hydrogen and Oxygen
If an electrical current is passed through water between electrodes (An electrode is a conductor used to establish electrical contact with a nonmetallic part of a circuit.), the water is split into its two parts: oxygen and hydrogen. This process is called electrolysis and is used in various industries, such as the manufacturing of metals like aluminum. If one of the electrodes is a metal, it will become covered or plated with any metal in the solution. This is how objects are silver-plated.
Before we get started, let’s do a quick review of some key chemistry terms – solution, solute, and solvent. A solution is a homogeneous mixture composed of two or more substances. The solute is the substance that is added and dissolved into the other substance. The solvent is what dissolves the solute. In this procedure, the salt is the solute, the water is the solvent. The salt and water mixed together is the solution.
Splitting Water Into Hydrogen and Oxygen
Equipment
Ingredients
- 30 inches 20 or 22 gauge, stranded, electrical wire
- 2 Number 2 pencils, sharpened
- 1 1/2 teaspoons table salt
- 1 piece cardboard
- Scissors
- Spoon or glass stir rod
- 12 ounces Warm water, not boiling
- 1 spool Electrical tape
- 1 9-volt battery
- Eye protection for each student
- A pair of wire strippers, if you have them. Otherwise, an adult will need to use the means available to them to strip away about an inch of the outer sheathing of the wire to expose the wires inside.
- Wire cutters
Instructions
-
Sharpen each pencil at both ends.

-
Cut the cardboard to fit over the glass. A 3” x 5” size works well.

-
Push the two pencils into the cardboard about an inch apart.

- Pour 12 ounces of warm water into a measuring cup, add the salt, and stir until the salt dissolves. Let it sit for about 3 to 5 minutes.
- Cut the wire into two 15" pieces using the wire cutters.
-
An adult needs to do this step. Strip about an of the outer sheathing of the wire from the wire, exposing the wires underneath. If you have a wire stripper, it's super easy. Otherwise, an adult will need to remove the sheathing.

-
Wrap one end of each piece of wire around the graphite pencil "lead". We are just trying to get it fitted properly. It will come unattached as you are trying to attach the other end to the battery. So, for now, we are just trying to get it formed to fit. Remove these from the pencil end.

-
Wrap one end (the end that was NOT wrapped around the pencil) of each wire to a terminal on the battery. Secure with electrical tape.

-
You will need a helper for this part. Place the cardboard holder with pencils into the salt water and attach the wires to the pencil lead. You can tape the wire in place on the graphite end of the pencils. The metal part of the pencils in the water should not touch. Do not touch the exposed end of any wires.

-
Check to make sure all connections are secure. How can you tell electricity is present, look at the pencil erasers. What is happening? What do you think is creating the bubbles?

Video
Conclusion
Through this electrolysis procedure, students were splitting water into its hydrogen and oxygen components, visible by the bubbles. We observed more hydrogen bubbles than oxygen bubbles.
What Happens When Splitting Water into Hydrogen and Oxygen?
In electrolysis, bonded elements are separated when an electric current passes through them. In this procedure we were splitting water that was in a solution with salt, resulting in the separation of the oxygen, hydrogen, sodium, and chloride. Let’s take a closer look at what happened.
In this procedure, salt (sodium chloride or NaCl) is dissolved in water. This makes the sodium chloride ions available in the water. We also have the hydrogen and oxygen that make up water. Remember, water is symbolized as H20 – there are two hydrogen atoms and one oxygen atom.
On the 9-volt battery, the smaller circular terminal is positive, and the larger hexagonal terminal is the negative contact.
The pencils are our electrodes once the wire is connected to the battery terminal and the graphite point on the pencil.
When the wires are attached to the battery and the metal on the pencils, electrons flow through the graphite in the pencil “lead” and into the solution.
As the electricity from the battery passes through electrodes (the pencils) and into the solution, the water and salt solution splits into hydrogen, oxygen, and chlorine gas. Remember, sodium chloride is salt. The chlorine gas comes from the sodium chloride – NaCl.
More bubbles come from the electrode connected to the negative terminal of the battery. These are hydrogen bubbles.
Fewer bubbles appear at the electrode attached to the positive terminal of the battery. These are mostly oxygen bubbles with some chloride gas bubbles. The chloride gas is a very tiny amount.
Through this electrolysis procedure, you are splitting water into its hydrogen and oxygen components, visible by the bubbles. We observed more hydrogen bubbles than oxygen bubbles.
The process is a bit more chemically involved. We explain in more advanced terms at the end of this section. Please use this explanation if it is age-appropriate for your student’s knowledge of chemistry.
Why Do We Use Salt When Splitting Water?
Why do we use salt in the water? Water by itself is not a very effective conductor of electricity. We use salt because it releases its own ions into the water that help conduct electricity.
Salt is NaCl – sodium chloride. Salt dissolves into the water, creating sodium and a small amount of chlorine gas.
The Chemistry Behind Splitting Water into Hydrogen and Oxygen
There is a more detailed process to explain how the oxygen bubbles form and why the hydrogen bubbles are bigger and more plentiful. Below we have the explanation. Use as applies to the student’s knowledge of chemistry.
The chemical equation for splitting water in this electrolysis procedure is:
energy (electricity) + 2 H2O -> O2 + 2 H2 .
When we have all wires attached to the battery and metal ends of the pencil, we have a flow of electrons.
On the negative electrode, the negative charge pushes out electrons into the water and salt (sodium chloride) solution. Then, as we expect, the positive end should accept those electrons so there is a strong flow of electrons.
However, water is not a very good conductor. The water molecules are split into a positively charged hydrogen ion and a negatively charged hydroxide ion. A hydrogen ion is a hydrogen atom without its electron. It is now positive.
Where did that electron go? The oxygen stole it! The oxygen more strongly attracts the electron from the hydrogen.
So, in the solution, we end up with a hydrogen ion (H+) and a hydroxide ion (OH-)
H2O -> H+ + OH-
The H+ picks up electrons and becomes a hydrogen atom with an electron. Two hydrogen bubbles combine and move to the surface as bubbles.
What happens to the hydroxide ion?
The hydroxide ion OH- is attracted to positively charged electrode. The electrode removes the stolen electron. The hydroxide ion mixes with three other hydroxide molecules to form 4OH-. This results in 1 molecule of oxygen and two molecules of water.
4 OH- > O2 + 2 H2O
We see the oxygen in the form of bubbles. But only one molecule of oxygen is created, which is why we see fewer bubbles on the anode side.
What happens to the two molecules of water that were formed? The water is released back into the solution too. A lot is going on when splitting water into its components via electrolysis!
Get the Splitting Water Printable Packet
Request the Printables
Enter your email below, and we’ll send you the templates and circuit printables. You will be added to our email community. If you’re already in our community, no problem! You may unsubscribe at any time by following the links in our emails. Thanks!
Related Resources You Might Like
How batteries work from Energizer
What’s Inside a Battery from Energizer
Making Simple Circuits and Light-Up Greeting Cards
Testing the Properties of Water
The Science of Electricity from the US Energy Information Service
Batteries, Circuits, and Transformers from the US Energy Information Service